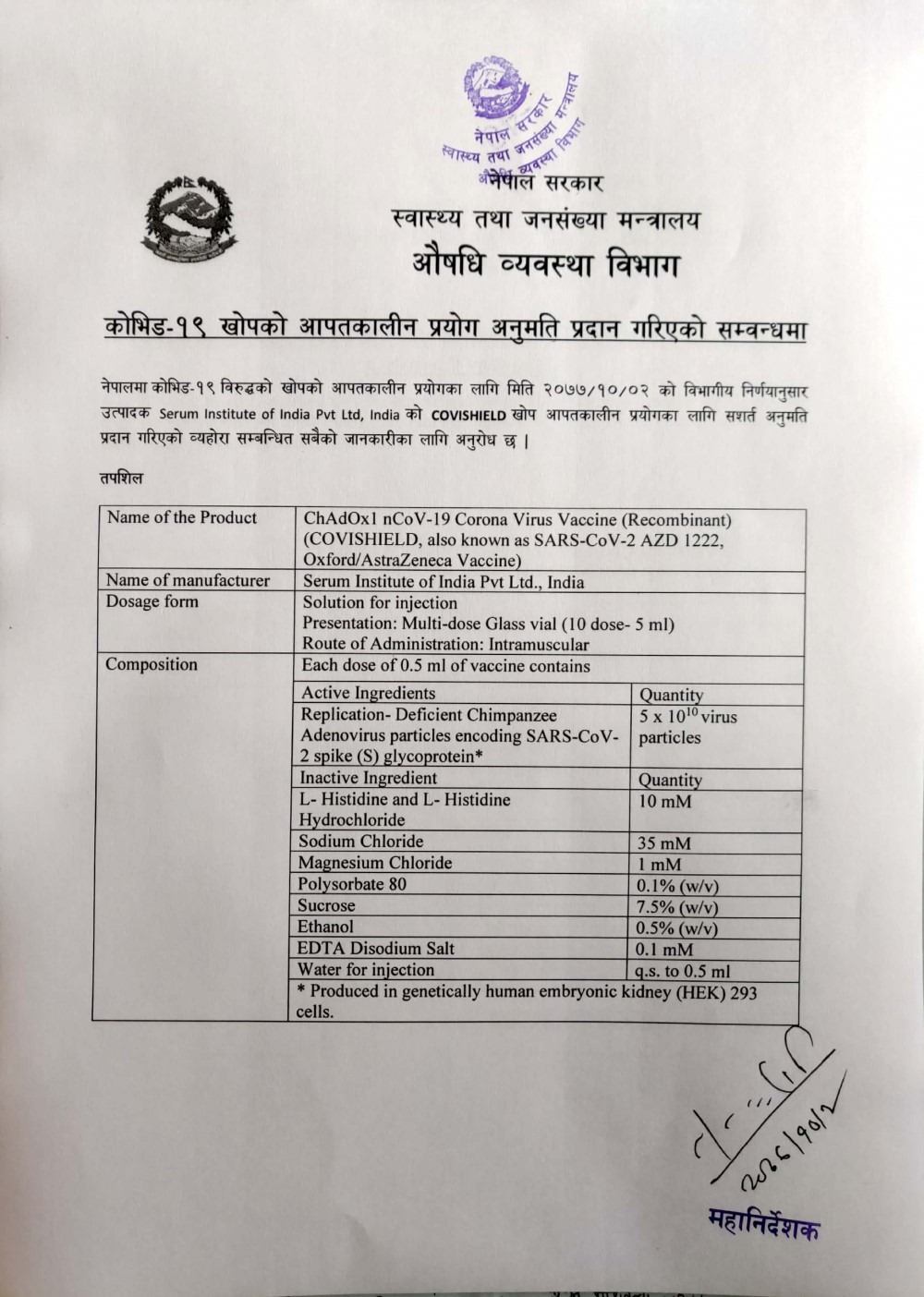
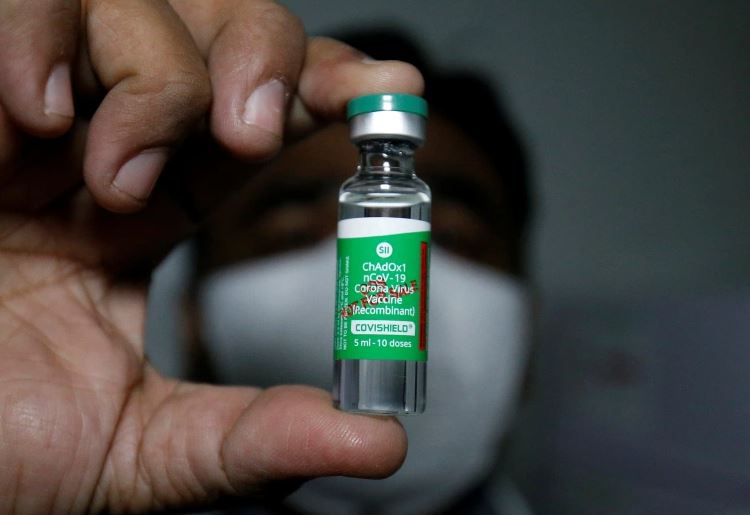
KATHMANDU: The Department of Drug Administration (DDA) has given permission for the emergency use of the coronavirus vaccine ‘Covishield’ in Nepal.
The vaccine produced by the Serum Institute of India has been given conditional permission for emergency use, the department said.
The department had two days ago called for the registration of vaccines to be used for emergency use.
India has already permitted the use of jabs developed by AstraZeneca with Oxford University and another vaccine by Bharat Biotech.








Comment