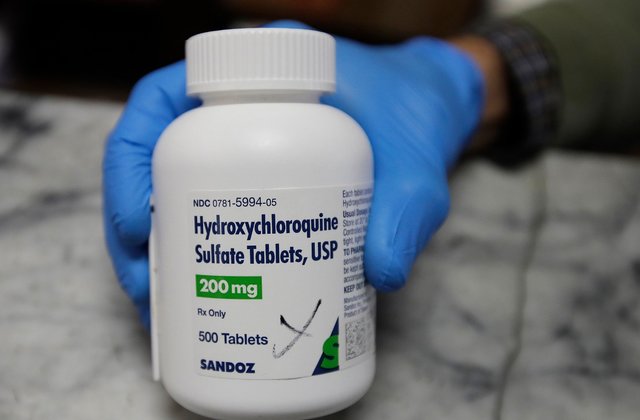
WASHINGTON D.C.: The National Institute of Health (NIH) on Thursday announced it has begun enrolling participants in a clinical trial to test the effectiveness of hydroxychloroquine, an anti-malarial drug, for treating COVID-19.
According to a report in The Hill, the first participants have enrolled in the trial at Vanderbilt University Medical Center in Nashville, Tennessee. US President Donald Trump has repeatedly referred to this drug in his briefings.
The Hill report stated that ‘participants will be randomly assigned to receive 400 mg of hydroxychloroquine twice daily for two doses (day one), then 200 mg twice daily for the subsequent eight doses (days two through five) or a placebo twice daily for five days.
On Wednesday, the US President thanked Prime Minister Narendra Modi over India’s decision on the export of hydroxychloroquine, in the wake of global coronavirus pandemic.
Last month, the Food and Drug Administration issued an emergency use authorization, allowing health care providers to use the medicine for illness.
(With inputs from agencies)








Comment