0%

The China-donated Covid-19 vaccine arrived in Nepal on 29th March 2021. Despite Nepal’s concern about the safety and efficiency of the Chinese COVID-19 vaccines promised to the government, the Chinese side has been exerting pressure on the government (through various channels) to use the vaccine.
The Chinese side had been pressuring, intimidating, and threatening Nepal to accept their vaccine.
Exerting Pressure: Nepal brought the vaccine after constant pressure from the Chinese Embassy in Kathmandu. China had warned Nepal saying if it fails to bring the vaccine immediately then they would send them to other countries, and that Nepal will have to be put on a much later list.
Also Read, China pressurizes Nepal to accept Sinopharm’s vaccine instantly
Intimidating: Later, China warned it would allow visas only to those Nepali applicants who have received the COVID-19 vaccine manufactured in China.
Issuing a press note on March 2, the Chinese Embassy in Kathmandu stated that the Nepali citizens visiting China should compulsorily submit a certificate of COVID-19 vaccination manufactured in China while applying for visas.
It is feared that this Vaccine politics would have a detrimental effect on global travel. If other vaccine-producing countries follow-suit, this will create hassles for the people to travel, particularly when Covid-19 has adversely impacted the travel and tourism industry.
Threatening: On February 5th, 2021, Chinese Foreign Minister Wang Yi spoke with his Nepali counterpart Pradeep Gyawali over the phone, mounting pressuring him to approve the vaccines first and then give them information about the vaccines; a turbulent approach to vaccine procurement.
It should be noted that the government has invested a considerable sum of money to get the 800,000 doses of the Sinopharm COVID-19 vaccine it received as aid from China. Nepal spent Rs. 21,810,300 on airfare to carry the vaccine that was given as a gift to the nation. The Ministry of Health and Population informed that the government-chartered a special airbus to bring in the COVID-19 vaccine after the China government did not allow its transport on a passenger plane.
The world has been questioning the effectiveness and safety of the China-manufactured Covid-19 vaccine. Nepal, too, has joined the list of those several countries around the world which have raised safety concern about the effectiveness of Chinese vaccine.
In Brazil, the third phase of the Sino-vac, produced by Sinopharm, was found to be only 50.4 percent effective which is comparatively less effective than all other vaccines.
The following graph shows the effectiveness of some renowned vaccines based on their success rate:
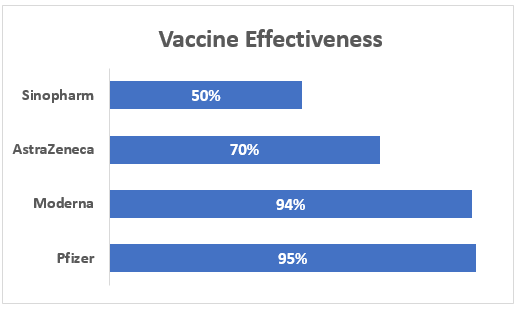
The Chinese vaccine is stated to be 50% effective, the Oxford vaccine (70% effective), and the Pfizer and Moderna vaccine (95% effective). Alongside, the people receiving the Chinese vaccine have complained of numerous side effects.
The Oxford vaccine despite being 70% effective has come under controversies in European Union nations regarding its side effects and efficiency, while the Sinopharm vaccine which is just 50% effective could induce more trouble than the solution.
Tao Lina, a Shanghai-based vaccine expert, posted a digital version of the manual for BBIBP-CorV, an inactivated vaccine developed by China National Biotec Group (CNBG), a subsidiary of China National Pharmaceutical Group Corporation, on Weibo on January 4. (Sinopharm). Due to the vaccine’s 73 local and systemic adverse reactions, he described it as an unsafe vaccine in his post.
However, the post was deleted on January 5 by China’s censors “due to violation of regulations.” According to Tao’s post, fever, fatigue, muscle ache, joint pain, cough, trouble breathing, nausea, diarrhea, and itchy skin are some of the most common side effects.
Dizziness, lack of appetite, vomiting, trouble swallowing, runny nose, constipation, and hypersensitivity are all listed as rare symptoms.
Likewise, Acute allergic reaction, lethargy, drowsiness, trouble sleeping, sneezing, nasopharyngitis, nasal congestion, dry throat, influenza, hypoesthesia, limb pain, palpitations, stomach ache, rash, skin and mucous membrane disorders, acne, eye pain, ear irritation, and lymphadenopathy are among the rare conditions mentioned.
Some lawmakers in the Philippines have questioned the government’s decision to purchase vaccines from Sinovac. Malaysian and Singaporean officials, who both requested doses from Sinovac, also had to inform their people that they would only authorize a vaccine if it proved to be safe and reliable.
Interestingly, on January 7, Tao apologized, saying that his sarcastic joke about vaccine instructions had been misinterpreted as genuine criticism, and vowed to set an example by taking two doses himself.
Therefore, what has to be understood is that while the Covid-19 was first seen in China, the doctors there warned the pandemic stating how dangerous it is.
Dr. Li Wenliang was the first one to raise the coronavirus’s alarm in the early days of the outbreak. Later police visited him and asked him to stop. It is now up to the Nepali people to decide whether to take it seriously or a mere jest.
If we look at the ASEAN nations, countries in the ASEAN alliance, have refused China’s offer to use its vaccine, and instead signed deals for the Pfizer vaccine and AstraZeneca vaccine, citing a lack of transparency in the production of the Chinese vaccine.
Some lawmakers in the Philippines have questioned the government’s decision to purchase vaccines from Sinovac. Malaysian and Singaporean officials, who both requested doses from Sinovac, also had to inform their people that they would only authorize a vaccine if it proved to be safe and reliable.
Therefore, it is high time that the government, as well as the citizens, needed to be aware of receiving these vaccines.